- What We Do
- Agriculture and Food Security
- Democracy, Human Rights and Governance
- Economic Growth and Trade
- Education
- Ending Extreme Poverty
- Environment and Global Climate Change
- Gender Equality and Women's Empowerment
- Global Health
- Water and Sanitation
- Working in Crises and Conflict
- U.S. Global Development Lab
- Antimcrobial Resistance
- Bedaquiline Donation Program
- Tuberculosis in India
The Challenge of MDR-TB
- MDR-TB is extremely difficult to fight and often costs people their health and their livelihoods.
- MDR-TB requires much longer treatment, often needing up to 2 years to complete a treatment course, compared to 6 months with drug-susceptible TB.
- Treatment options are lengthy, toxic, and complicated, involving hundreds of pills and injections, and carry severe side effects such as hearing loss.
- Treatment cost per case can increase as much as 100-fold.
- MDR-TB has significantly lower cure rates. Only 52 percent of MDR-TB patients have a successful treatment outcome.
- In 2015, 250,000 people died of MDR-TB(including Rifampicin resistant).
- If left untreated, a person with MDR-TB may infect 10–15 people every year.
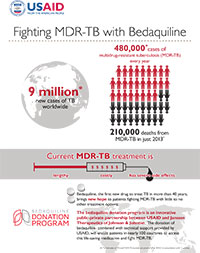
Antimicrobial resistance (AMR) is a growing global threat. AMR occurs everywhere in the world today, compromising our ability to combat infectious diseases and undermining advances in health and medicine. Multidrug-resistant tuberculosis (MDR-TB) is one example of AMR that is jeopardizing many of the gains we have made against tuberculosis (TB) in recent years.

The global burden of MDR-TB remains very high. In 2015, there were 580,000 cases of MDR-TB and 250,000 deaths (including Rifampicin resistant). When patients with MDR-TB are left untreated, or fail to complete their treatment properly, they transmit resistant forms of TB back into the community and can infect others. This in turn threatens the effective prevention and treatment of TB, making the disease more difficult and potentially impossible to treat.
Bedaquiline Offers New Hope to MDR-TB Patients
Because MDR-TB is highly contagious and notoriously difficult to treat, it is critical to develop innovative solutions to curb this epidemic. Bedaquiline, developed by USAID's partner Janssen Pharmaceuticals (of parent company, Johnson & Johnson), is the first new class of antibiotics that has been U.S. Food and Drug Administration (FDA) approved in more than 40 years. The use of this drug in combination with existing second-line drugs provides new hope for MDR-TB patients with limited treatment options.
However, the cost of bedaquiline makes it difficult to access, especially for low- and middle-income countries. To solve this problem and get the drug to those who need it most, Janssen will donate 30,000 6-month courses of bedaquiline over the next 4 years – a $30 million value. To maximize the use of bedaquiline to treat MDR-TB, the U.S. Agency for International Development (USAID) is serving as the implementing partner, providing technical assistance to strengthen recipient countries' health systems.
Countries Participating in the Bedaquiline Donation Program.
Countries highlighted on the map represent countries that have reached out to the Global Drug Facility to access bedaquiline through the donation program.
- Armenia
- Bangladesh
- Belarus
- Bolivia
- Burma
- Cameroon
- Congo, Democratic Republic of
- Cote d'Ivoire
- Djibouti
- Dominican Republic
- Ethiopia
- France
- Georgia
- Haiti
- India
- Indonesia
- Liberia
- Kazakhstan
- Kenya
- Korea, Republic of
- Kyrgyzstan
- Lesotho
- Moldova, Republic of
- Namibia
- Netherlands
- Niger
- Nigeria
- Pakistan
- Papua New Guinea
- Peru
- Philippines
- Swaziland
- Tanzania, United Republic of
- Thailand
- Uganda
- Turkmenistan
- Uzbekistan
- Vietnam
More Resources
- USAID Bedaquiline Infographic [PDF, 452KB]
- Learn more about the USAID-Janssen Bedaquiline Donation Program.
- Find more information on bedaquiline from the World Health Organization [PDF, 1.8MB].
- Read the USAID-Janssen Bedaquiline Donation Press Release
- Share tweets and other social media with the Bedaquiline Social Media Toolkit [PDF, 645KB].







Comment
Make a general inquiry or suggest an improvement.