- What We Do
- Agriculture and Food Security
- Democracy, Human Rights and Governance
- Economic Growth and Trade
- Education
- Ending Extreme Poverty
- Environment and Global Climate Change
- Gender Equality and Women's Empowerment
- Global Health
- Water and Sanitation
- Working in Crises and Conflict
- U.S. Global Development Lab
 Photo credit: Jane Silcock/USAID
Introduction
Photo credit: Jane Silcock/USAID
Introduction

At a Glance
- Introduction
- Scope of the Problem
- Multi-Sectoral Nutrition Strategy
- How Nutrition Impacts Child Mortality
- How Nutrition Impacts Maternal Mortality
- Conclusion
View the PDF [PDF, 275KB].
Good nutrition is essential to reducing maternal and child mortality around the world and reaching the U.S. Agency for International Development (USAID) goals for preventing child and maternal deaths. USAID's maternal and child survival efforts are focused within 25 countries1 that together represent over 70 percent of maternal and child deaths worldwide, and USAID prioritizes interventions that have the largest impact on mortality. Building on the experience and evidence garnered in the past two decades of reductions in child and maternal deaths, USAID's efforts in this area aim to accelerate progress in order to save the lives of 15 million children and nearly 600,000 women by 2020 (USAID, 2014a). With undernutrition estimated to be an underlying cause of 45 percent of child mortality and anemia contributing to 20 percent of maternal mortality, investing in nutrition is fundamental to achieving USAID's maternal and child survival goals (Bhutta, et al., 2013; Black, et al., 2008). Figure 1 provides a breakdown of undernutrition's contribution to disease-specific deaths among children under 5.2 Nutrition interventions are among the lifesaving interventions that can have the greatest impact in preventing child and maternal deaths (USAID, 2014a).
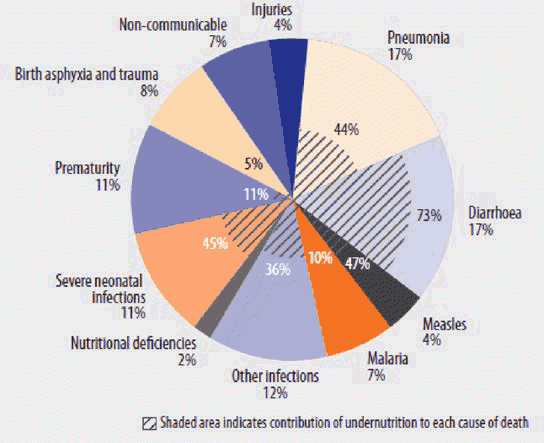
Scope of the Problem
The Lancet 2013 Series on Maternal and Child Nutrition highlighted the significant contribution of undernutrition to child mortality, stemming from fetal growth restriction, stunting, wasting, micronutrient deficiencies, and suboptimal breastfeeding. In 2011, 3.1 million children died as a result of undernutrition (Black, et al., 2008). In 2013, at least 161.5 million children experienced stunted growth and 50.8 million suffered from acute malnutrition (UNICEF, 2015). Globally, approximately 13 percent of women were estimated to be undernourished, and 38 percent of all pregnant women suffered from anemia (Black, et al., 2008; Stevens, et al., 2013). Furthermore, micronutrient deficiencies – in particular vitamin A, zinc, iodine, and iron – are estimated to affect more than 2 billion people worldwide, with adverse effects that include premature death, poor health, blindness, stunting, reduced cognitive development, and low productive capacity (Bhutta, et al., 2013).
Multi-Sectoral Nutrition Strategy
USAID’s Multi-Sectoral Nutrition Strategy (2014–2025) reflects a vision for 2025 of a world in which countries, communities, and families have the capacity to achieve and sustain healthy, well-nourished populations. This vision includes reduced rates of childhood stunting and wasting and improved nutrition for women leading to millions of lives daved, increased resilience, and significant benefits to broad-based economic growth and development. To this end, the Multi-Sectoral Strategy sets forth a comprehensive approach to integrate nutrition across USAID's work in maternal and child health, adolescent health, agriculture, livelihoods, water, sanitation and hygiene (WASH), HIV and AIDS, family planning, and humanitarian assistance, bringing together a variety of funding streams and approaches across the agency, with nutrition at the nexus.
This brief intends to share the evidence on the links between nutrition-specific interventions and child/maternal mortality, laying the foundation for the larger series of programmatic guidance briefs to follow. These briefs will provide:
- case examples and additional details related to the implementation of successful nutrition activities and programs
- evidence on non-mortality outcomes such as impaired cognitive development in children
- in-depth analysis of linkages with other nutrition-sensitive technical areas such as agriculture, family planning, and WASH
How Nutrition Impacts Child Mortality
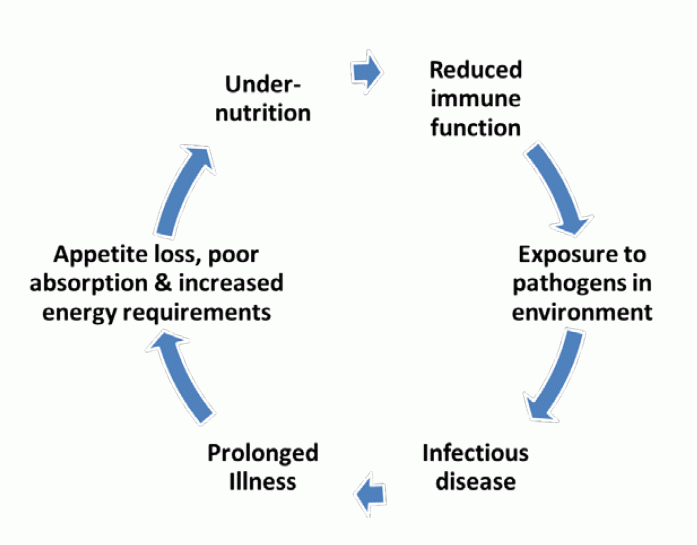
Undernutrition is an underlying cause of more than 3 million child deaths per year (Bhutta, et al., 2013). Undernutrition has a direct effect on child mortality as it compromises immune function, increases susceptibility to infectious diseases, and hastens the progression, severity, and duration of disease. It is also a consequence of poor health, as infectious diseases (e.g., diarrhea, acute respiratory infections, TB, HIV) increase energy requirements and often reduce appetite and nutrient absorption (see figure 2) (Bhutta, et al., 2013; WHO, 2013). Poor sanitation and hygiene contribute to fecal pathogens in the environment which, when ingested through contaminated food and water, lead to disease. While often ignored, poor family planning practices, such as short birth intervals and early pregnancy, also increase the risk of small for gestational age births, undernutrition, and mortality (Rutstein, 2008). Inadequate maternal nutrition during pregnancy and suboptimal infant feeding are likewise key risk factors. Child undernutrition is the leading contributor to the burden of disease in sub-Saharan Africa and fourth in South Asia (UNSCN, 2014; Lim, et al., 2012). While the immediate causes of child death often "mask" the synergistic and bidirectional effects of undernutrition and disease, undernutrition remains one of the most important underlying determinants of child mortality (Bhutta, et al., 2013). For example, epidemiologic studies show that a severely stunted child has a four times higher risk of mortality and a severely wasted child has a nine times higher risk (UNICEF, 2013a). Timely nutrition-specific3 interventions, when implemented at critical points in the lifecycle, can have a significant impact in reducing child mortality globally if taken to scale in high-burden countries. The Lancet 2013 Series on Maternal and Child Nutrition identified 10 evidence-based interventions that, if scaled to 90 percent coverage in 34 focus countries, could reduce child mortality by 15 percent,4 in addition to reducing stunting by 20 percent and severe wasting by 60 percent (Bhutta, et al., 2013). These interventions target pregnant women and children during the critical '1,000 day window of opportunity' from pregnancy to 2 years of age. The 10 interventions, included in table 1, are reflected in the USAID Multi-Sectoral Nutrition Strategy and fall into four broad categories: maternal nutrition during pregnancy (including iron and folic acid, calcium, and energy protein supplementation), infant and young child feeding practices (early and exclusive breastfeeding, and appropriate complementary feeding with foods prepared and provided after hand washing with soap), micronutrient supplementation for children, and management of acute malnutrition. The economic benefits of scaling up these nutrition interventions in 40 low income countries are large, with a return of US$16 for every US$1 invested (IFPRI, 2014).
There is ample experimental and observational evidence surrounding each of the interventions listed in table 1. Poor maternal nutrition contributes to poor fetal development and low birth weight, and an estimated 60–80 percent of neonatal deaths occur in low birth weight babies (UNICEF, 2013a). Adequate peri-conceptional folic acid supplementation reduces the risk of neural tube defects in the child by 72 percent (Bhutta, et al., 2013). Since 2001, the World Health Organization (WHO) has recommended exclusive breastfeeding up to 6 months (WHO, 2001). Infants not exclusively breastfed are 15 times more likely to die from pneumonia and 11 times more likely to die from diarrhea than children who are exclusively breastfed (UNICEF, 2013a).5 Micronutrient supplementation is one of the simplest and most cost-effective public health interventions. For example, a review of 43 randomized control trials demonstrated that vitamin A supplementation reduced all-cause mortality by 24 percent and diarrhea-related deaths by 28 percent in children aged 6–59 months (Bhutta, et al., 2013). Appropriate management of acute malnutrition, including use of ready-to-use therapeutic and supplementary foods combined with safe drinking water, can avert 435,000 child deaths per year according to The Lancet Series analysis (Bhutta, et al., 2013; UNICEF, 2013b). In addition, well-designed nutrition-sensitive interventions can complement the impact of nutrition-specific activities by addressing the underlying determinants of malnutrition.
How Nutrition Impacts Maternal Mortality
Optimal maternal nutrition is an important contributor to the survival of both the mother and child and promotes women's overall health, productivity, and well-being.Current evidence shows that there are two critical pathways through which women's nutrition affects survival outcomes: anemia and calcium deficiency. The strongest evidence is around anemia, a condition in which a person's blood has too few red blood cells or hemoglobin to transport oxygen to cells in the body.6 Maternal anemia is estimated to contribute to 20 percent of maternal deaths (Black, et al., 2008). Severe anemia, when hemoglobin levels are less than 7.0 g/dl, presents a significant risk of mortality for women of reproductive age, whether or not they are pregnant (Ronsmans, Collins, & Filippi, 2008). Pregnancy increases the risk of maternal anemia (specifically iron deficiency anemia) as there is an increase in maternal iron requirements to support both maternal and fetal needs (Steer, 2000). The risks associated with anemia increase as hemoglobin levels decrease. Very severe anemia can lead to heart failure and death from shock. In one study performed in Kenya, women of reproductive age with severe anemia were eight times more likely to die than those with higher hemoglobin levels (Ronsmans, Collins, & Filippi, 2008). There is growing evidence that anemia is linked to increased blood loss during delivery and puts women at greater risk of postpartum hemorrhage (Kavle, et al., 2008). Postpartum hemorrhage is responsible for 25 percent of maternal deaths globally and is the leading cause of maternal mortality (MCHIP, 2015; Say, 2014). With high levels of anemia among pregnant women in many of USAID's maternal and child survival priority countries (52 percent in South Asia and 56 percent in Central and West Africa), and issues with access to health care in these settings, the necessity of preventive actions for anemia is clear (Stevens, et al., 2013). There are also significant co-morbidities such as malaria infection and shorter birth intervals that interact with anemia and increase the risk of mortality.
Interventions that increase iron uptake and stores, reduce blood loss and infection, and address other micronutrient deficiencies could prevent at least half of all anemia cases. In high-risk settings, emphasis must be placed on improving maternal iron status both during and prior to pregnancy, so the mother's iron stores are sufficient before her body is subjected to the additional demands of pregnancy. Iron supplementation is a very effective intervention for reducing the risk of anemia in women, both pregnant (70 percent reduction in risk of anemia at term) and non-pregnant (27 percent reduction) (Bhutta, et al., 2013). During pregnancy in particular, the high requirements for iron are difficult to reach through diet alone, and WHO recommends that in areas of high levels of iron deficiency, women should receive 6 months of daily iron supplementation during pregnancy (Klemm, et al., 2011). Nutrition-sensitive interventions that increase dietary diversity and improve regular consumption of iron-rich food sources and iron-fortified foods are also important interventions for maintaining iron levels throughout the life cycle.
Epidemiological studies have linked low calcium intake to gestational hypertension — a condition of high blood pressure during pregnancy that can lead to pre-eclampsia and eclampsia, now the second most important cause of maternal mortality worldwide (Black, et al., 2013).7 Calcium supplementation during pregnancy prevents pre-eclampsia in communities with low calcium intake (Ronsmans, Collins, & Filippi, 2008). Supplementation reduces pre-eclampsia risk by 55 percent and preterm birth risk by 24 percent as well as reducing the risk of fetal growth restriction (Bhutta, et al., 2013).
Lastly, there is some initial evidence on the beneficial maternal health effects of vitamin A, zinc, and multiple micronutrient supplementation as well as emerging interest around adolescent nutrition. For example, vitamin A may improve maternal immunity and reduce anemia (Ronsmans, Collins, & Filippi, 2008). However, more research is necessary to establish causal links with maternal mortality reduction or any other effects on maternal and child health. Similarly, only limited rigorous research has been done to explore the relationship between maternal anthropometry and mortality, though there is some evidence that maternal underweight is associated with maternal mortality, including increased risk of difficult labor and the need for Cesarean section (Black, et al., 2013; UNICEF, 2013b). Maternal undernutrition, both stunting and underweight, is strongly associated with an increased risk of infants born small for gestational age. Maternal obesity is associated with maternal, neonatal, and infant death as well as complications including gestational diabetes, pre-eclampsia, hemorrhage, infection, and birth trauma (Black, et al., 2013).
Conclusion
Scaling up high impact nutrition interventions is essential to preventing maternal and child deaths. Integrating nutrition-specific interventions into maternal and child health programs should be a priority for USAID health projects. Optimal maternal and child nutrition in the first 1,000 days helps ensure healthy mothers and newborns and good growth and development for infants and children. It also decreases vulnerability to infectious diseases and the negative cycle of disease and undernutrition that leads to child death. There is a global consensus on the efficacy of these critical high-impact and cost-effective maternal and child nutrition interventions. Programs and missions will need to consider context-specific implementation challenges and cost-effectiveness, which will be explored in the accompanying implementation guidance briefs in this series.
Table 1: Nutrition-Specific Interventions to Reduce Child and Maternal Mortality
| Interventions* | Effects on Disease and Mortality* | Illustrative Actions** |
|---|---|---|
| Child Nutrition8 | ||
| Promotion of early and exclusive breastfeeding up to 6 months; continued breastfeeding through 24 months and longer along with appropriate and timely introduction of adequate and safe complementary foods.9 | Reduces risk of diarrheal disease and acute respiratory infections. Reduces neonatal/child mortality. Improves immune function. |
Comprehensive social and behavior change interventions, including counseling and support at facility and community levels. Growth monitoring and promotion to detect and respond to faltering. Implement Baby-Friendly Hospital Initiative. Implement International Code of Marketing of Breastmilk Substitutes. Protection of maternity benefits. Responsive/Active feeding. |
| Appropriate complementary feeding education or provision. | Reduces risk of infectious disease and neonatal/child mortality. |
Comprehensive social and behavior change interventions, including counseling and support at facility and community levels. Complementary foods should support continued breastfeeding, start at 6 months of age, and include a variety of nutrient-rich foods given in amounts, frequency, and consistency to cover the nutritional needs of the growing child. Use of multiple micronutrient powders for home fortification of foods consumed by infants and young children 6–23 months, when appropriate. |
| Vitamin A supplementation for children 6–59 months of age. | Reduces mortality, improves immune function, reduces severity of illness, reduces risk of visual impairment, and allows faster recovery from infectious disease such as measles, malaria, and diarrhea. | Vitamin A supplementation campaigns in vulnerable population; and along with treatment for measles, diarrhea, and acute malnutrition. |
| Zinc supplementation for children 12–59 months of age. | Improves immune function. Reduces severity of diarrheal disease. Improves recovery from various infectious diseases. Reduces mortality in children under 5. | Zinc supplementation to children with diarrhea. Should be paired with oral rehydration solution for diarrhea. |
| Management of moderate acute malnutrition. | Reduces mortality in children under 5. | Screening/measurement, identification, and treatment of malnutrition, e.g., supplementary feeding |
| Management of severe acute malnutrition. | Reduces mortality in children under 5. | Screening/measurement, identification, and treatment of malnutrition, e.g., therapeutic feeding, community management of acute malnutrition, or integrated management of acute malnutrition. |
| Maternal Nutrition | ||
| Multiple micronutrient supplementation in pregnancy. | Reduces incidence of low birth weight, small for gestational age births, preterm births. Promotes early childhood growth. Reduces maternal anemia. Reduces mortality in children under 5. | Multiple micronutrient supplementation for pregnant women, per country guidelines and may include vitamin A, B1, B2, B6, B12, niacin, folic acid, vitamin C, vitamin D, vitamin E, copper, selenium, iodine, iron, and zinc.10 |
| Calcium supplementation to mothers at risk of low intake. | Reduces gestational hypertension, pre-eclampsia, preterm birth, and fetal growth restriction. Reduces maternal mortality. | Calcium supplementation for pregnant women. |
| Balanced energy protein supplements as needed. | Reduces fetal growth restriction, small for gestational age, still births, low birth weight, and adverse perinatal outcomes. | Provision of supplements, with approximately 25 percent of total energy as protein.2 |
|
Peri-conceptional folic acid supplementation or fortification. Iron supplementation for women of reproductive age or pregnant women. |
Reduces risk of neural tube defects, low birth weight, anemia, and mortality in children under 5. Reduces maternal anemia, low birth weight, and neonatal mortality. |
Intermittent iron and folic acid supplementation in menstruating women. Daily supplementation with iron and folic acid for women during pregnancy. Intermittent iron and folic acid supplementation for non-anemic pregnant women (1, 2, or 3 non-consecutive days/week). Fortification of cereals and other foods. |
| Global | ||
| Increased dietary diversity.11 | Reduces micronutrient deficiencies | Increase variety and quantity of micronutrient rich foods, including animal source foods that enhance absorption of micronutrients. |
| Wheat and maize flour fortification. | Reduces micronutrient deficiencies | Industrial fortification in countries where industrially produced flour is consumed by large population groups |
*Drawn from The Lancet 2013 Series on Maternal and Child Nutrition unless otherwise indicated
** Drawn from WHO Essential Nutrition Actions (2013) unless otherwise indicated.
This technical brief will be periodically updated. Comments from readers are welcome to Laura Itzkowitz, especially comments to help clarify the information provided or where additional information may be useful (last updated October 16, 2015).
References
Bhutta, Z.A., et al. (2013). . Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? The Lancet Maternal and Child Health Series 382 (9890), 452-477.
Black, R.E., et al. (2008). Maternal and child undernutrition: global and regional exposures and health consequences. The Lancet Maternal and Child Health Series 371 (9608), 243–260.Black, R.E., et al. (2013). Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet 382 (9890), 427-451.
Caulfield, L.E., de Onis, M., Blössner, M., & Black, R.E. (2004). Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 80(1):193-8.
Grantham-McGregor, S., et al. (2007). Developmental potential in the first 5 years for children in developing countries. The Lancet 369(9555): 60-70.
International Food Policy Research Institute (IFPRI). (2014). Global nutrition report 2014: actions and accountability to accelerate the world's progress on nutrition. Washington, DC.
Kavle, J.A., et al. (2008). Association between anaemia during pregnancy and blood loss at and after delivery among women with vaginal births in Pemba Island, Zanzibar, Tanzania. J Health Popul. Nutr 26(2): 232–240.
Klemm, R.D., et al. (2011). Are we making progress on reducing anemia in women? Cross-country comparison of anemia prevalence, reach, and use of antenatal care and anemia reduction interventions. A2Z: The USAID Micronutrient and Child Blindness Project.
Lim, S., et al. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380, 2224-2260.
Maternal and Child Health Integrated Program (MCHIP). (2015). Prevention of postpartum hemorrhage.
Ronsmans, C., Collins, S., & Filippi, V. (2008). Maternal mortality. In: Semba, R., Bloem, M., eds. Nutrition and Health in Developing Countries. The Humana Press. Totowa, NJ.
Rutstein, S.O. (2008). Further evidence of the effects of preceding birth intervals on neonatal, infant, and under five years mortality and nutritional status in developing countries: evidence from the Demographic and Health Surveys [PDF, 503KB]. Accessed January 4, 2015.
Say, L., et al. (2014). Global causes of maternal death: a WHO systemic analysis. The Lancet Global Health Blog, 2:e323-33.
Steer, P.J. (2000). Maternal hemoglobin concentration and birth weight. American Journal of Clinical Nutrition, 71(5 supp) (1285s-1287s).
Stevens, G.A., et al. (2013). Global, regional and national trends in hemoglobin concentration and prevalence of total and severe anemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. The Lancet Global Health Blog. 1:e16-25.
United Nations Children's Fund (UNICEF). (2013a). Improving child nutrition: the achievable imperative for global progress.
UNICEF. (2013b). Ready-to-use therapeutic food for children with severe acute malnutrition. UNICEF Position Paper No. 1, June 2013.
UNICEF. (2015). Child nutrition interactive dashboard.
United Nations Standing Committee on Nutrition (UNSCN). (2014). Nutrition and the post-2015 Sustainable Development Goals: a technical note [PDF, 540KB].
U.S. Agency for International Development (USAID). (2014a). Acting on the call 2014 [PDF, 14.2MB].
Victora, C.G., Adair, L., Fall, C., Hallal, P.C., Martorell, R., Richter, L., & Sachdev, H.S. (2008). Maternal and child undernutrition: consequences for adult health and human capital. The Lancet, 371(9609): 340-357.
World Health Organization (2009). Global health risks: mortality and burden of disease attributable to selected major risks [PDF, 3.6MB].
World Health Organization (WHO). (2013). Essential nutrition actions 2013.
Footnotes
1. Afghanistan, Bangladesh, Democratic Republic of the Congo, Ethiopia, Ghana, Haiti, India, Indonesia, Kenya, Liberia, Madagascar, Malawi, Mali, Mozambique, Burma, Nepal, Nigeria, Pakistan, Rwanda, Senegal, South Sudan, Tanzania, Uganda, Yemen, and Zambia.
2. The most recent analysis dates from 2004 (Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. (WHO, 2009). Updated figures by disease are not currently available.
3. Nutrition-specific interventions address the immediate determinants of undernutrition (e.g., exclusive breastfeeding, dietary supplementation). Nutrition-sensitive interventions address the underlying and systemic causes of undernutrition (e.g., WASH, agriculture, women's empowerment).
4. Since undernutrition is an underlying rather than direct cause of mortality, nutrition interventions have the potential to reduce it only in part. Additionally, the scale-up included in the calculation for this potential reduction does not reach 100 percent coverage, and while the ten proven interventions may have a significant impact on reducing undernutrition and mortality, they do not represent the full gamut of available and effective interventions for undernutrition.
5. Frequent episodes of diarrhea in the first 2 years of life increase the risk of stunting and mortality and can impair cognitive development. At the same time, undernourished children have weakened immune systems, which make them more susceptible to enteric infections and lead to more severe and prolonged episodes of diarrhea (Caulfield, 2004; Grantham-McGregor, et al., 2007; Victora, et al., 2008). For more information on the relationship between WASH and nutrition, including environmental enteropathy, please refer to the the WASH & Nutrition Technical Implementation Brief, which is part of this series.
6. The causes of anemia are multifactorial. Anemia is a clinical condition that can be caused by low iron consumption, malaria infection, intestinal worms, genetic disorders (thalassemia, sickle cell), or other infectious diseases (e.g., HIV, TB). While iron deficiency is thought to be the most common cause of anemia globally, other micronutrient inadequacies, including folate, vitamin B12 and vitamin A, can also lead to anemia. More broadly, all causes of anemia can be classified into three groups: (1) blood loss (e.g., bleeding, NSAID consumption, gastrointestinal tract conditions, childbirth, menstruation, helminths); (2) a decrease of faulty red blood cell production (e.g., iron deficiency anemia, vitamin deficiencies, genetic disorders, bone marrow and stem cell production disorders); (3) destruction of red blood cells (e.g., genetic disorders, toxins from advanced liver and kidney disease, hemolytic disease in newborns and pregnant women, clots, malaria).
7. Pooled analysis showed that calcium supplementation during pregnancy reduced risk of pre-eclampsia by 55 percent and that of severe pre-eclampsia by 25 percent (Black, et al., 2013).
8. Many of these interventions may be included in a broader IMCI or ICCM platform.
9. For additional guidance.
10. Drawn from Cochrane Review.
11. This intervention is not listed in The Lancet Nutrition Series or WHO Essential Nutrition Actions but there is a consistent evidence base around increased dietary diversity.






Comment
Make a general inquiry or suggest an improvement.